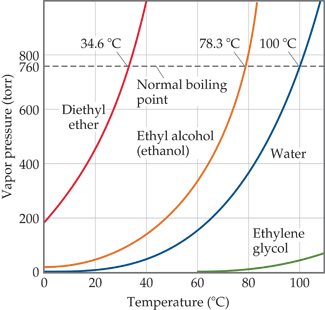
Is a 50/50 mixture of acetone and water an azeotrope? Also, why does the first drop of destillate form at 40C? - Quora

EP0183110B1 - Azeotrope-like compositions of trichlorotrifluoroethane, ethanol, acetone, nitromethane and hexane - Google Patents

Preparation and properties of low boiling point of alcohol and acetone-based magnetic fluid - ScienceDirect
ChemIDplus - 67-64-1 - CSCPPACGZOOCGX-UHFFFAOYSA-N - Acetone [NF] - Similar structures search, synonyms, formulas, resource links, and other chemical information.

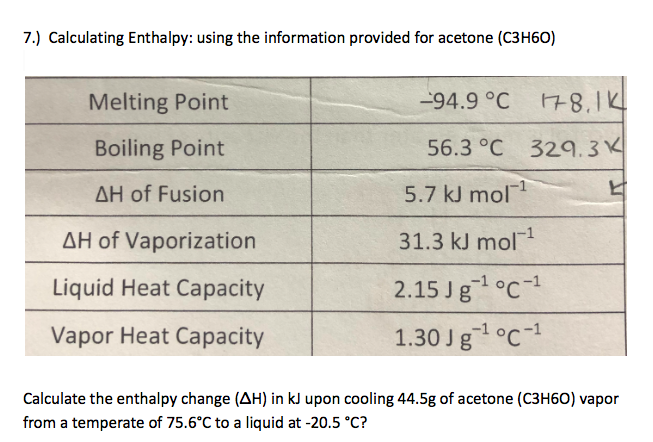
Acetone, or propanone, is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is … | Chemical structure, Chemistry, Molar mass
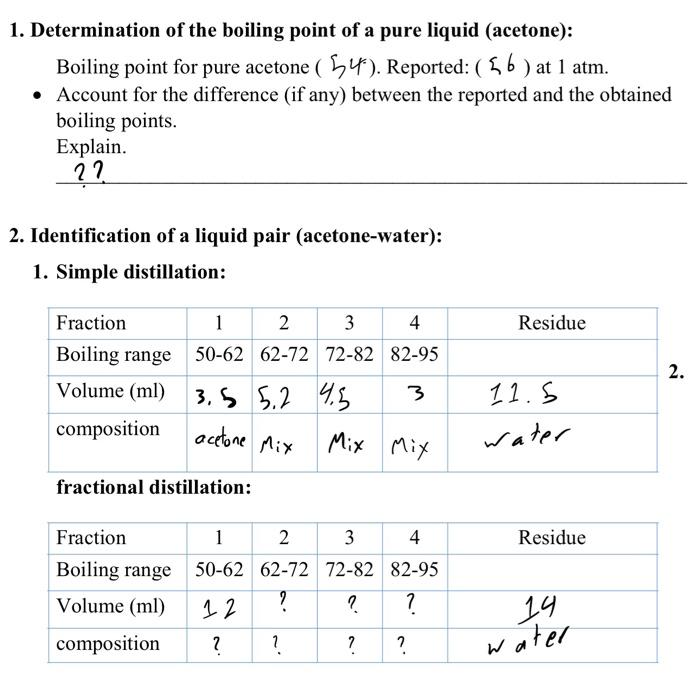
How to calculate the boiling point of a mixture made up of 2 liquids ( acetone+water in the same ratios) - Quora
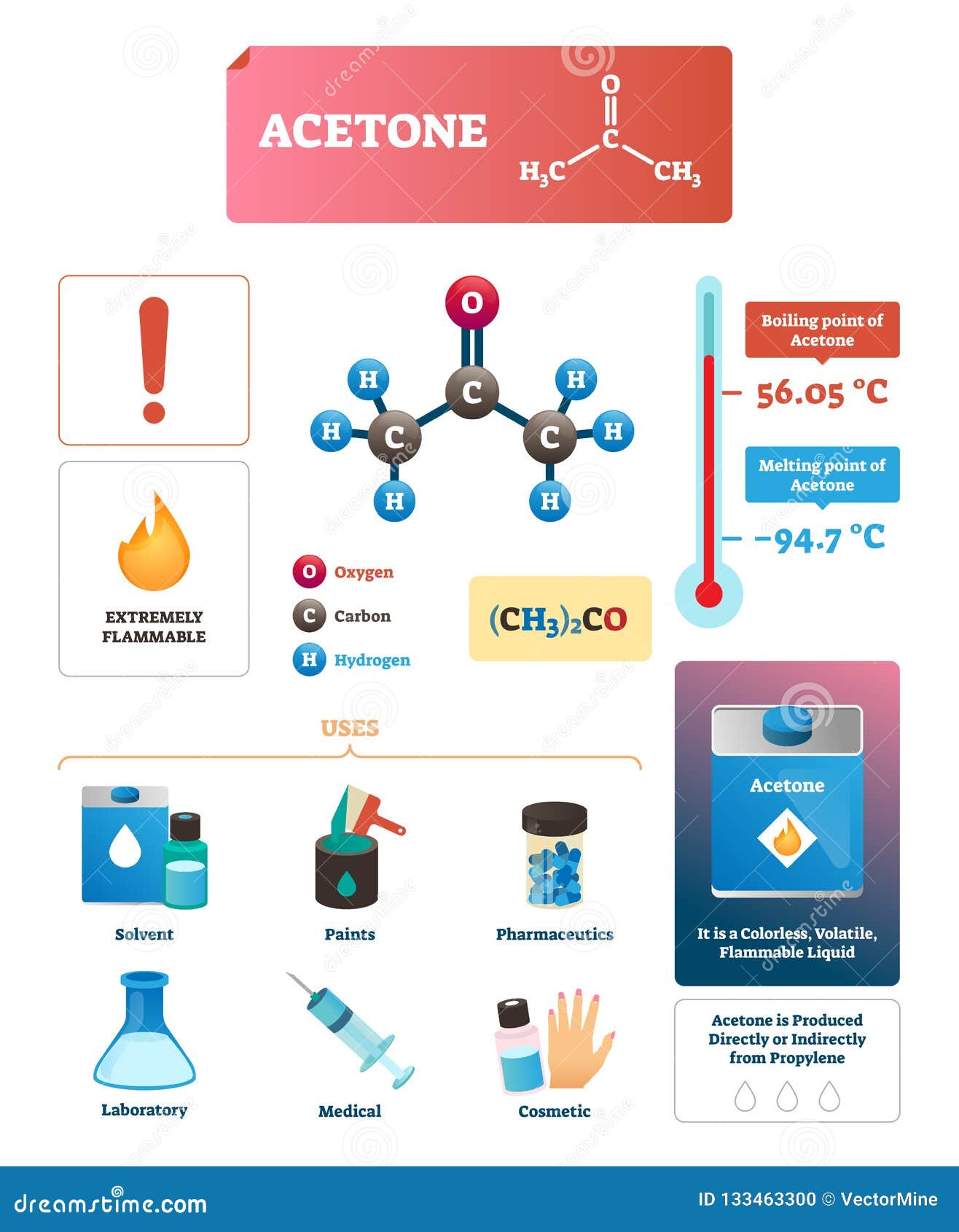
Acetone Vector Illustration. Chemical and Physical Explanation Infographic Stock Vector - Illustration of educational, medical: 133463300

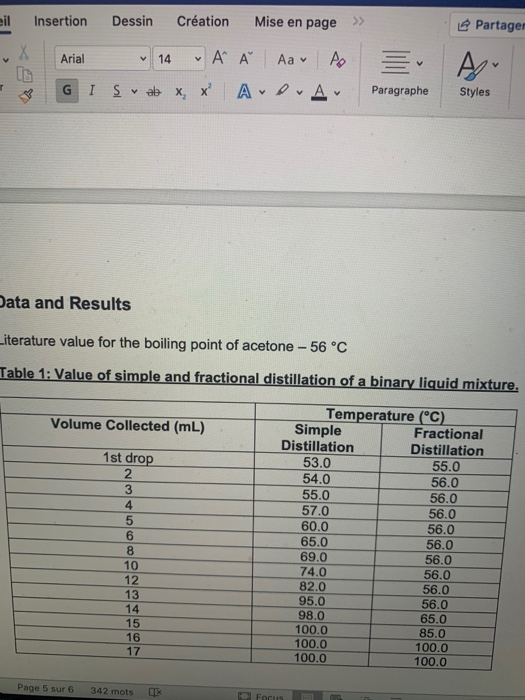
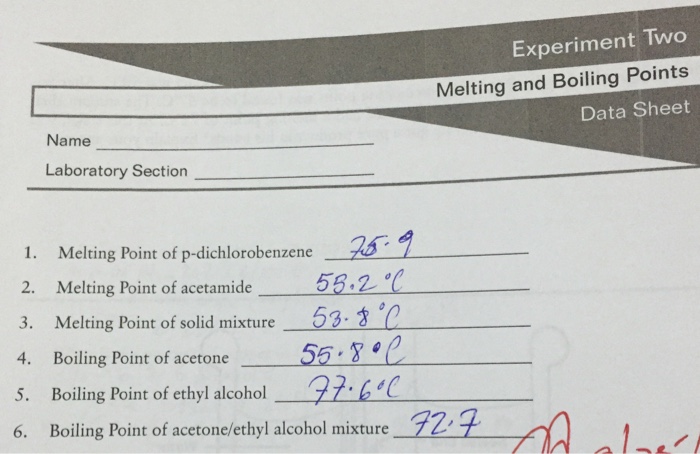
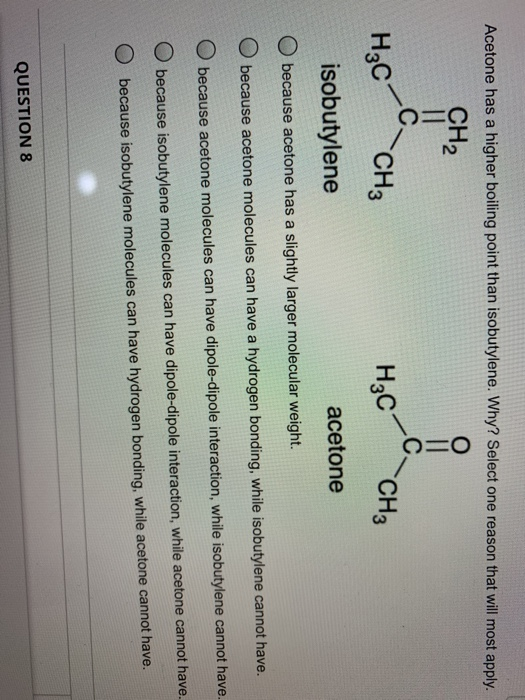

![PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar PDF] Separation of Water-Acetone Mixture Using Suitable Entrainer (Simulation) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3b24cb3fdaba29b0182f3943cf35a512430d1196/29-Table3-2-1.png)