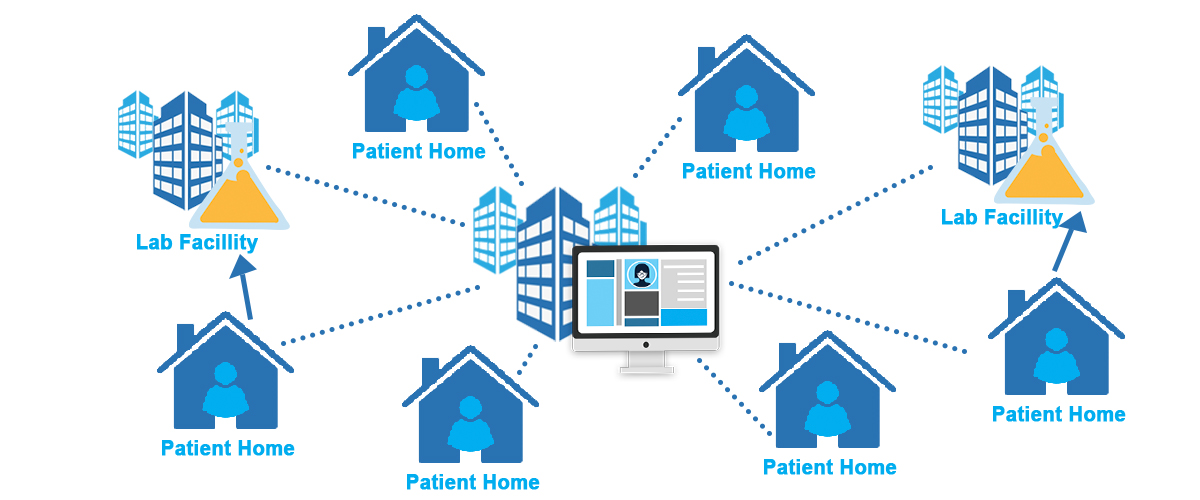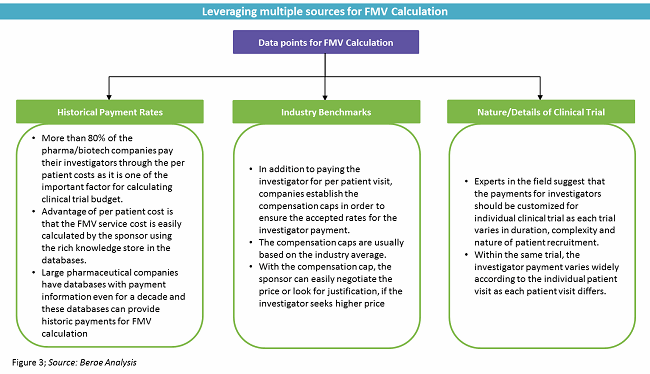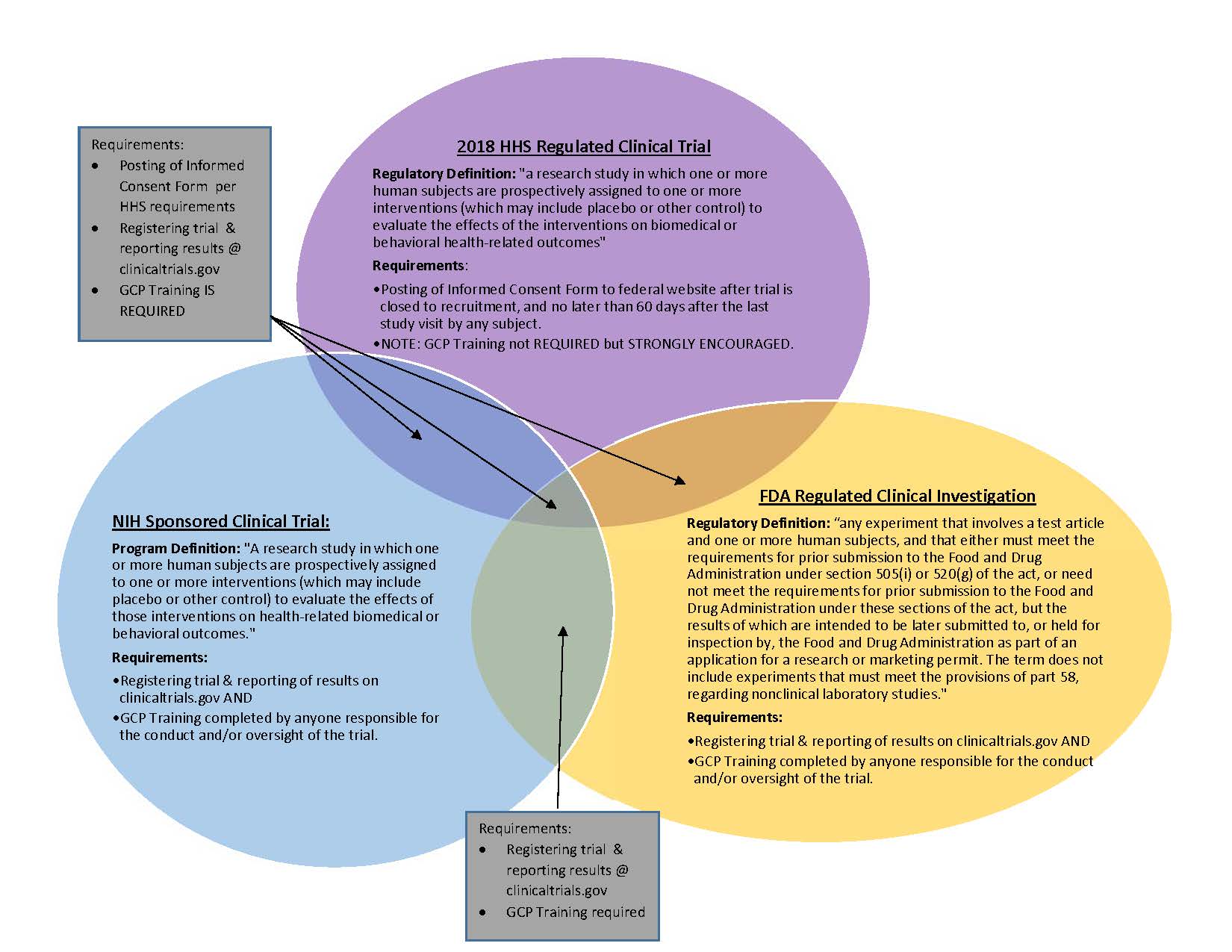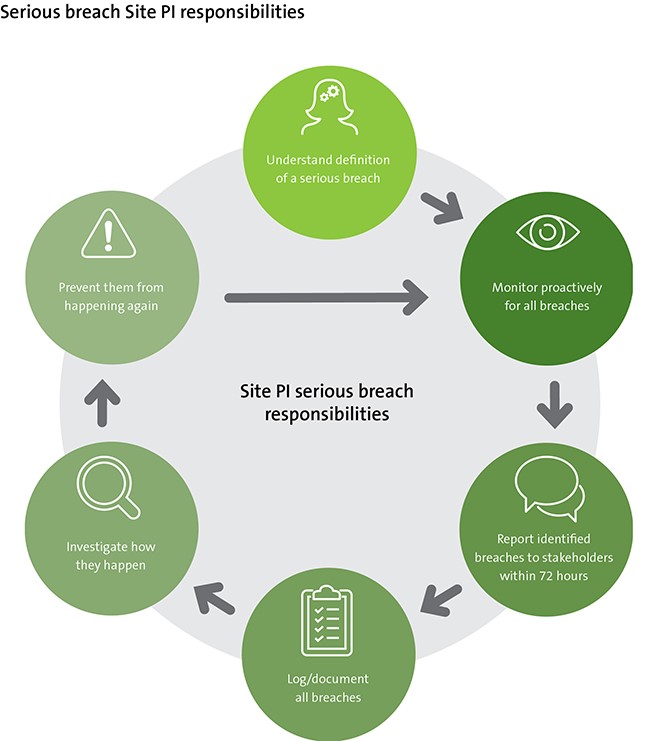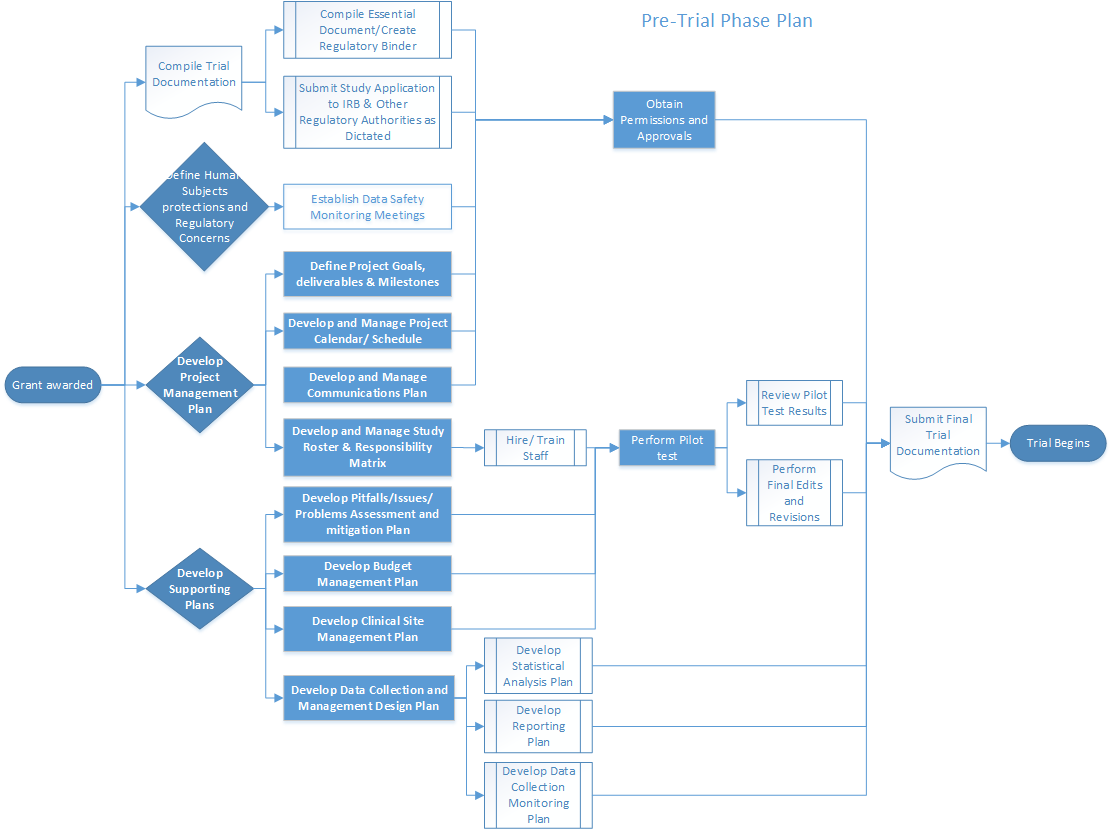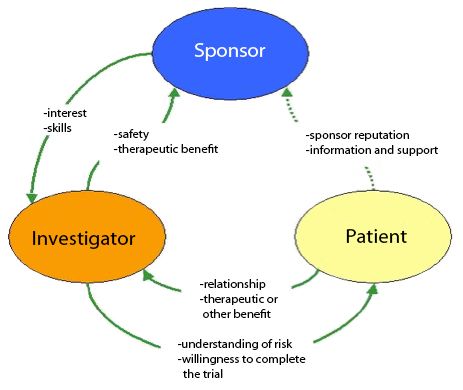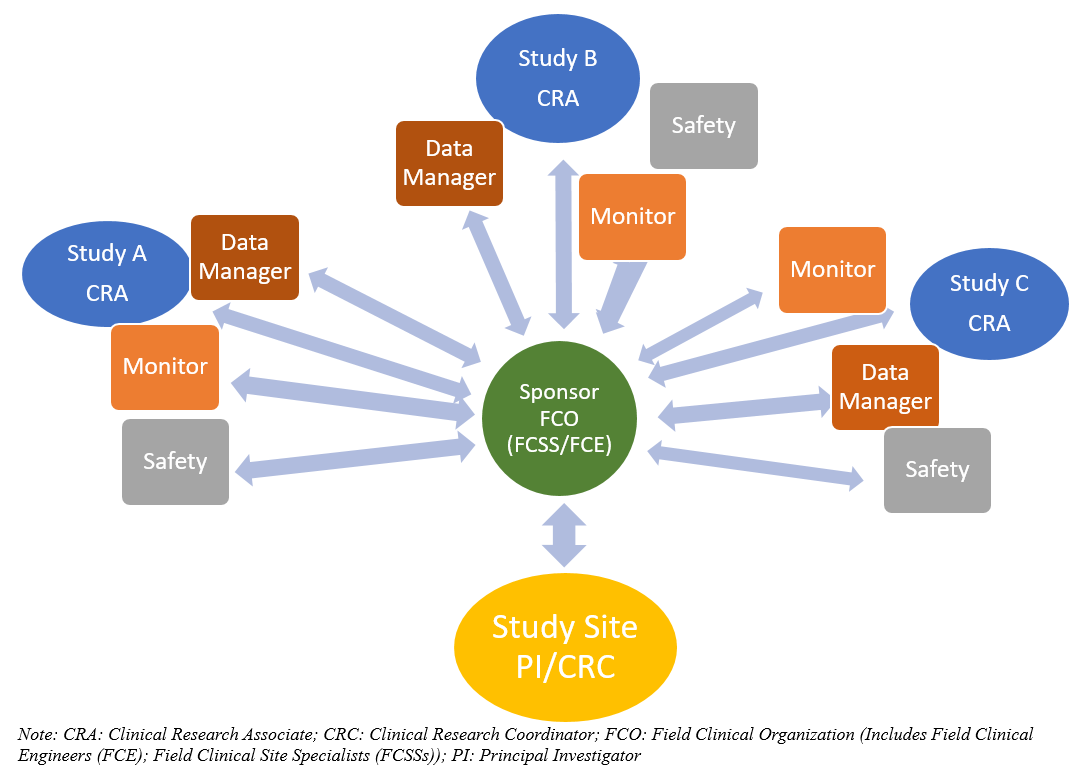
Sponsor-Site Communication in Device Trials: Evolution of a Dedicated Field Clinical Organization Throughout Study Execution - ACRP

Frontiers | The Current Status and Future Direction of Clinical Research in Japan From a Regulatory Perspective

EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download
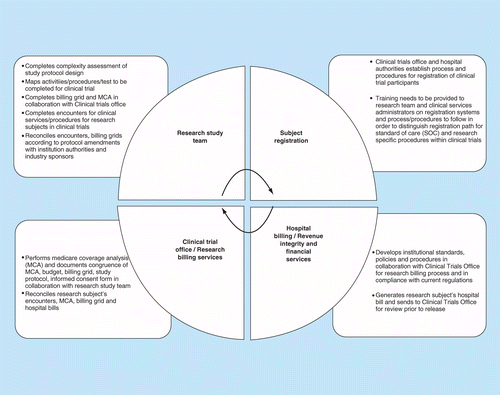
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA
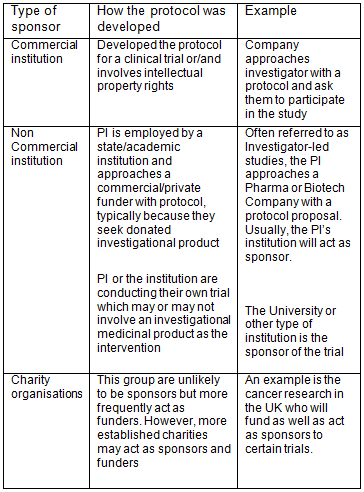
![PDF] Sponsor-investigator-relationship: challenges, recent regulatory developments and future legislative trends | Semantic Scholar PDF] Sponsor-investigator-relationship: challenges, recent regulatory developments and future legislative trends | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/cebe49113140c5242f8a80e21c7ff4cf13329a03/4-Table4-1.png)
PDF] Sponsor-investigator-relationship: challenges, recent regulatory developments and future legislative trends | Semantic Scholar