The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials - The Lancet
CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials | PLOS Medicine

Figure 1 from Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. | Semantic Scholar

Assessment of the reporting quality of randomized controlled trials related to the pharmacotherapy of COVID-19 based on the CONSORT 2010 checklist: a systematic review - Clinical Microbiology and Infection

CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials | The BMJ
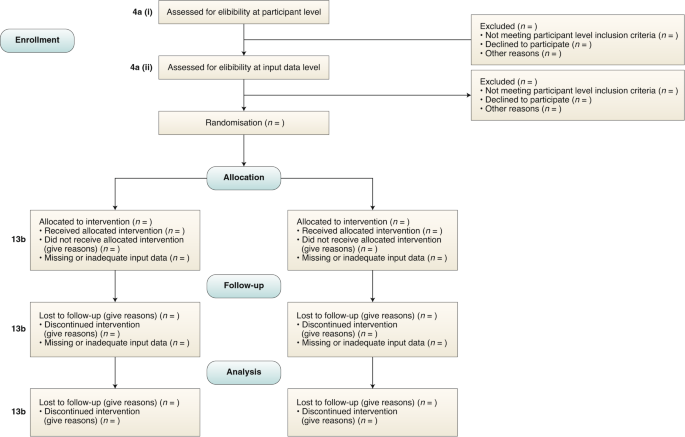
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine

CONSORT Diagram for the Randomized Clinical Trial of the Power Through... | Download Scientific Diagram

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension - ScienceDirect

JCI - Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy

Table 1 from Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. | Semantic Scholar
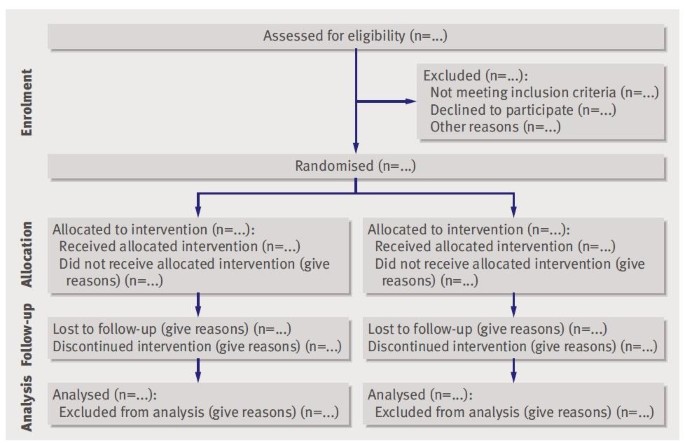
CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials | Trials | Full Text

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension - The Lancet Digital Health








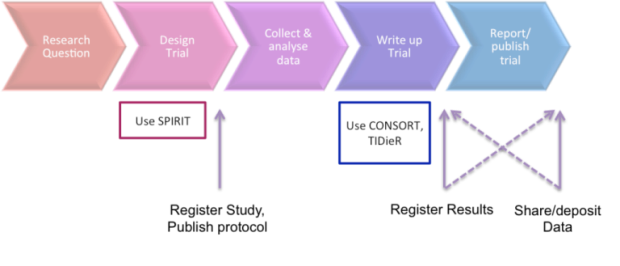



%202015%20%20Flow%20diagram.jpg)