Clinicaltrialsregister.eu ▷ Observe Clinical Trials Register News | EU Clinical Trials Register - Update

Clinical Trial Naming: Using The EU Clinical Trials Register To Create Differentiation - Six Degrees

The Current Status of European and National Financial Sources for Clinical Research and Their Impact on Paediatric Non-commercial Clinical Trials: A Case Study of the Czech Republic | SpringerLink
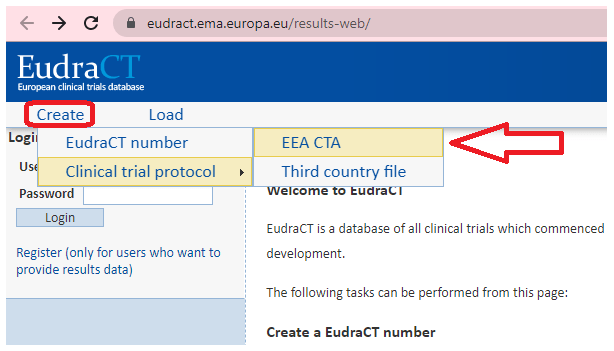
What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? - Sofpromed

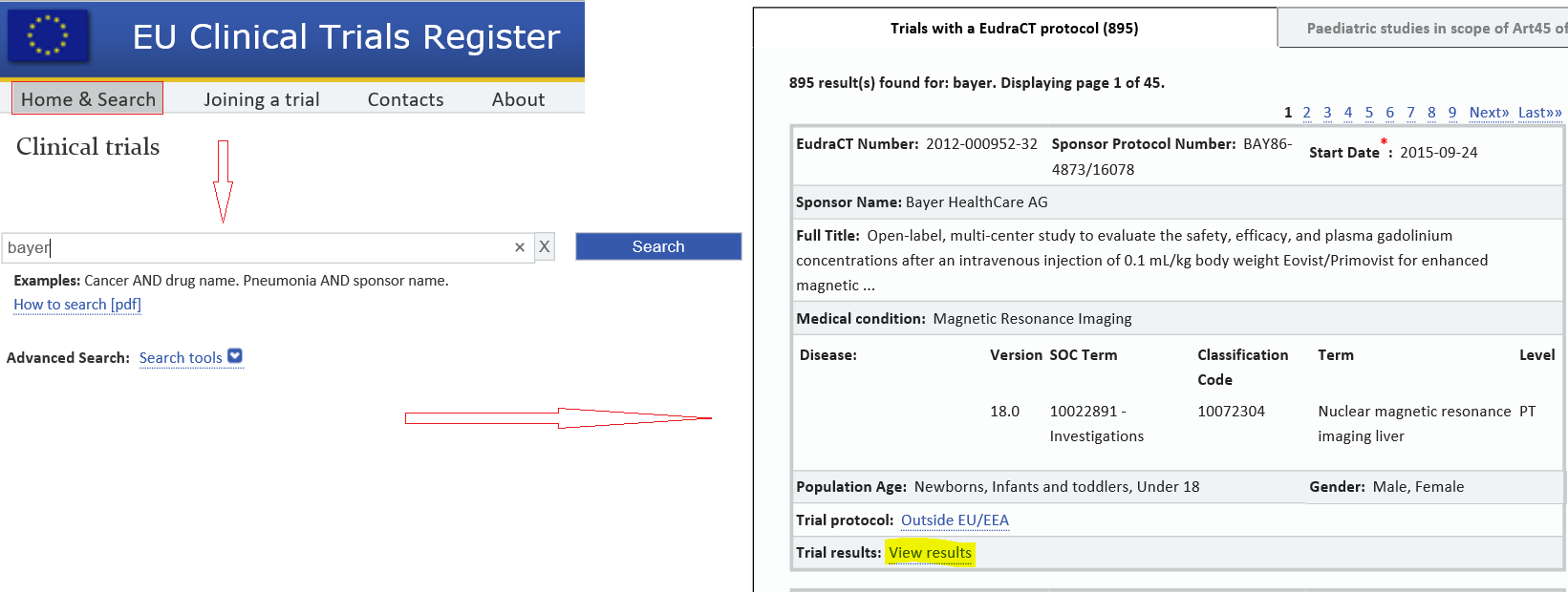
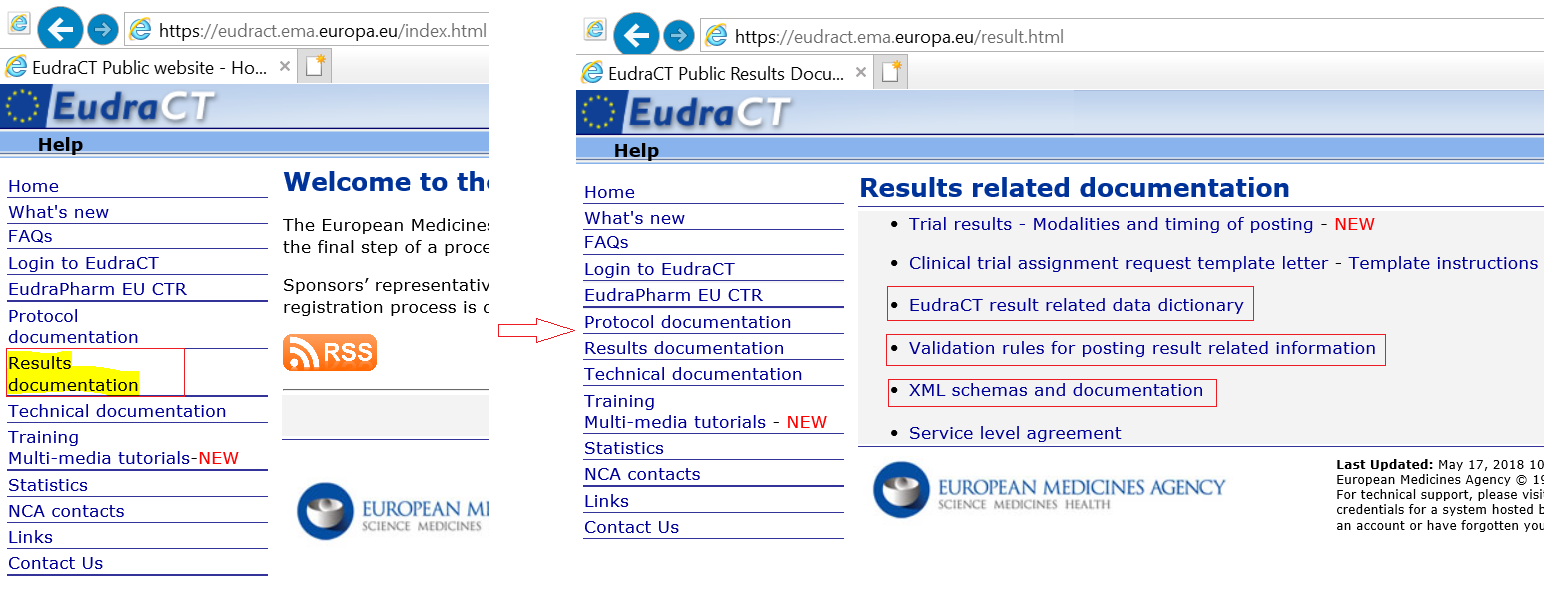
EU Clinical Trial Registry: small industry and academia lack of compliance in reporting Clinical Trial Applications results