FDA Regulation of Neurological and Physical Medicine Devices: Access to Safe and Effective Neurotechnologies for All Americans - ScienceDirect
Wednesday June 2, 2016 FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C

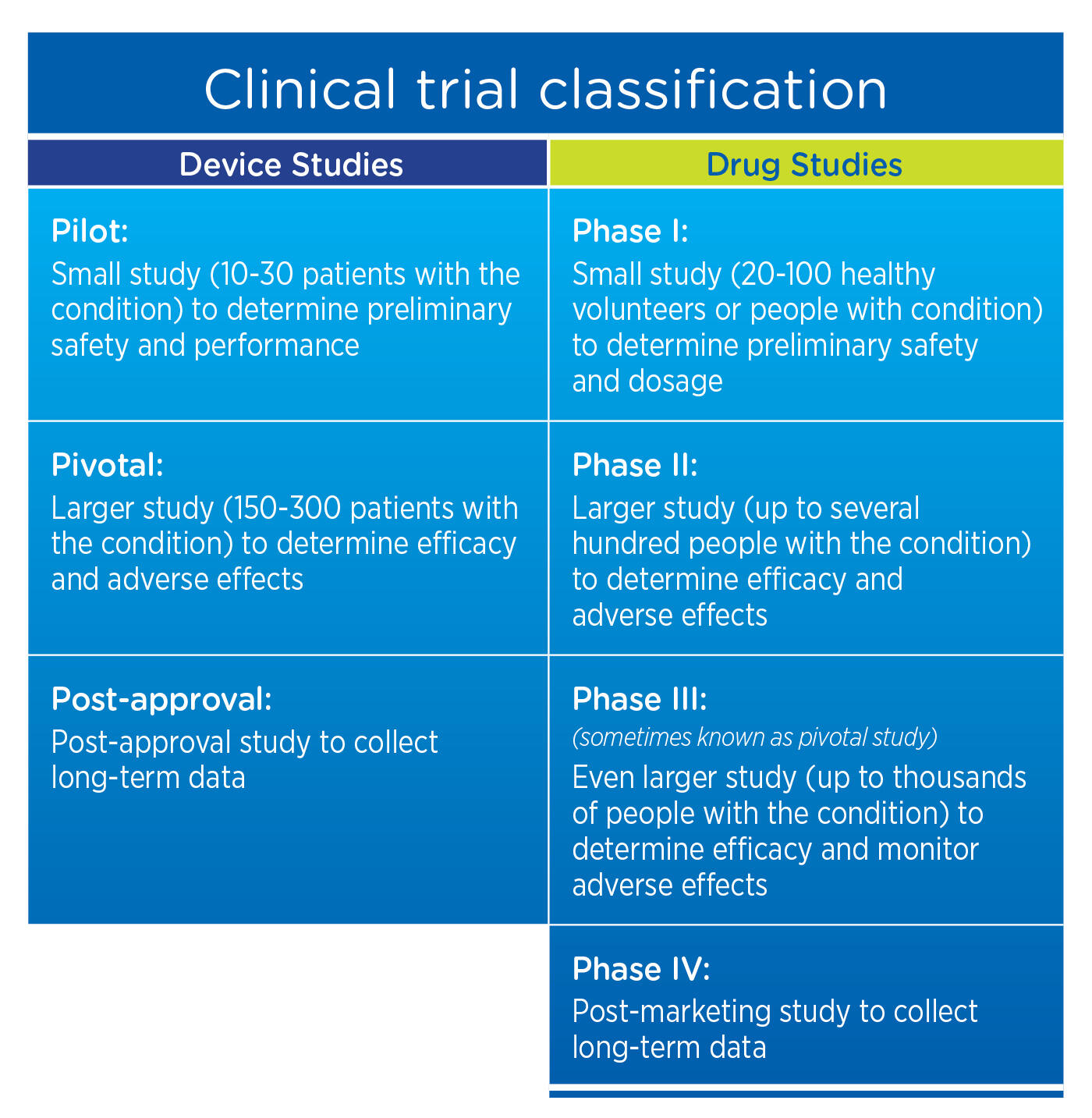
Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices | JACC: Basic to Translational Science

Updated FDA Guidance Helps Device Study Sponsors Better Anticipate Coverage for Investigational Devices | Advisories | Arnold & Porter
Guidance on Research Involving FDA-Regulated Investigational Articles: Investigational Drugs and Medical Devices
Live Case Presentations During Investigational Device Exemption (IDE) Clinical Trials - Guidance for Institutional Review Boards
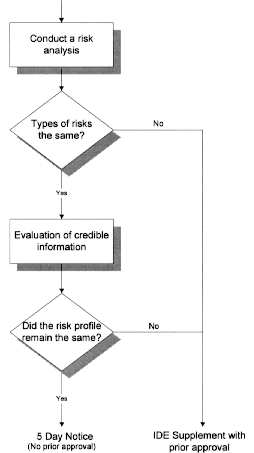
Guidance and Procedures: Use of Devices in Clinical Research and Brief Overview Investigational Device Exemption Applications (