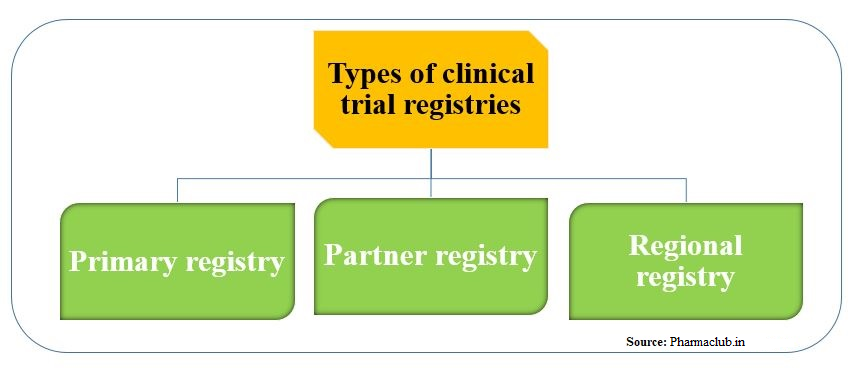
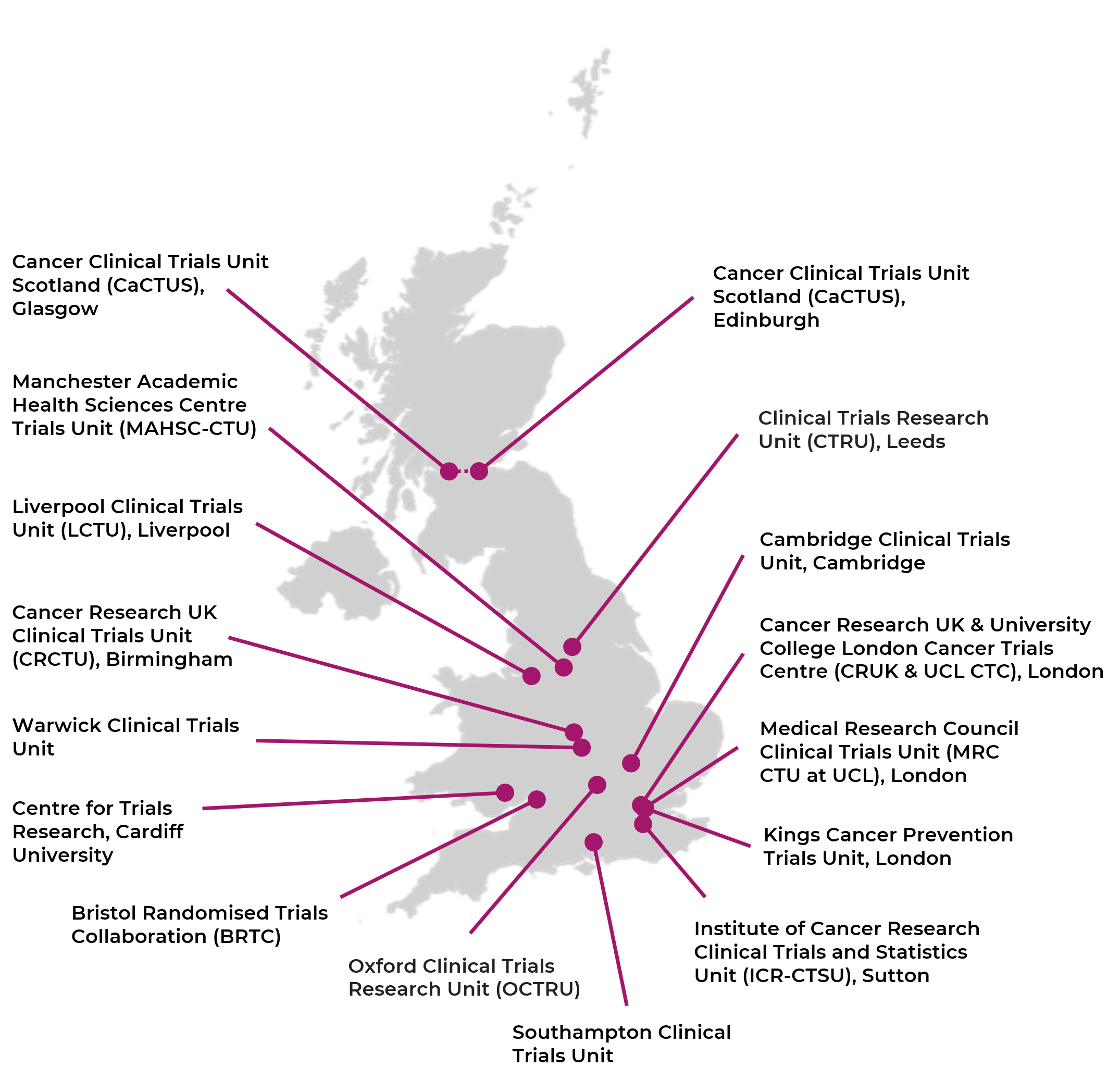
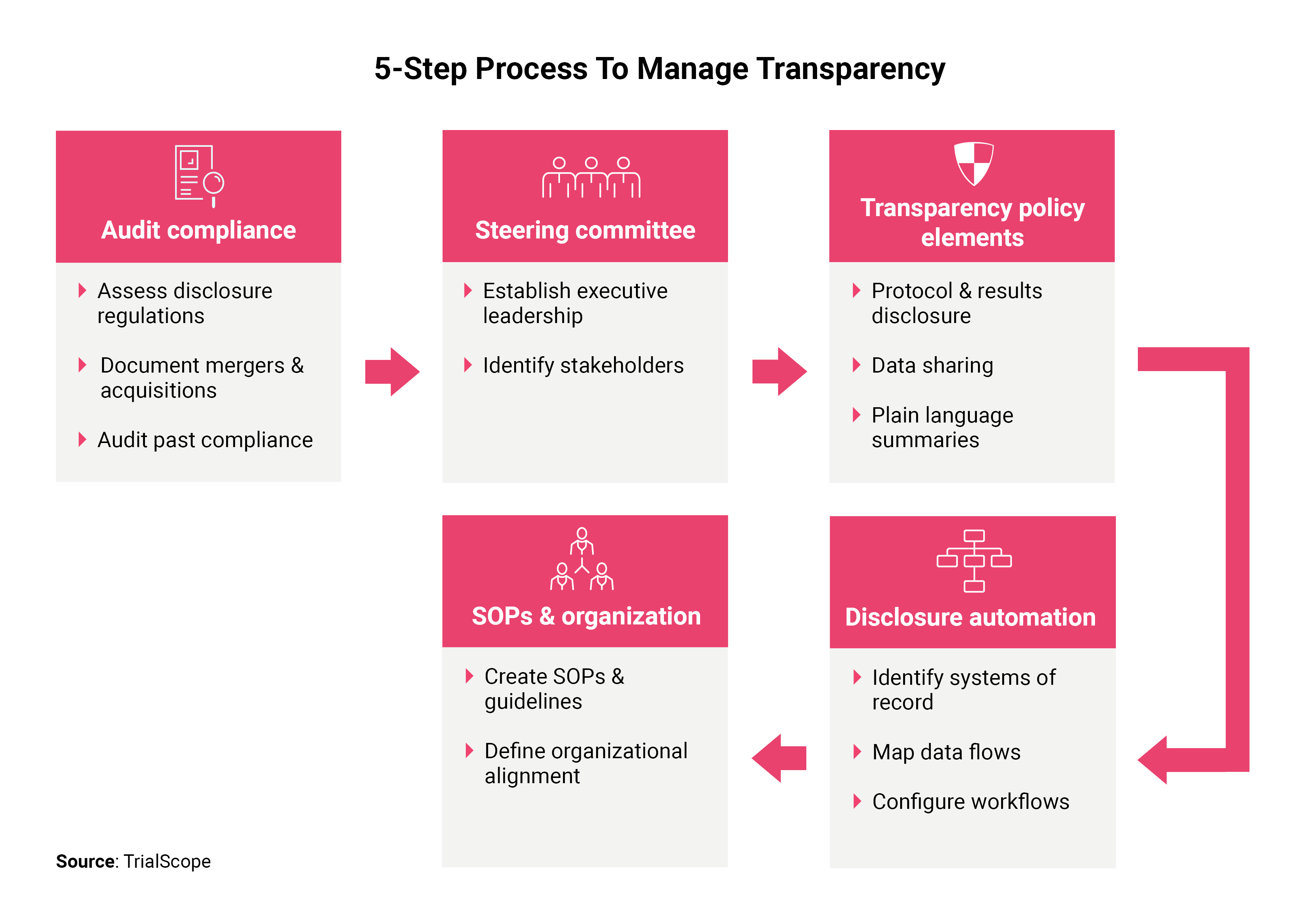
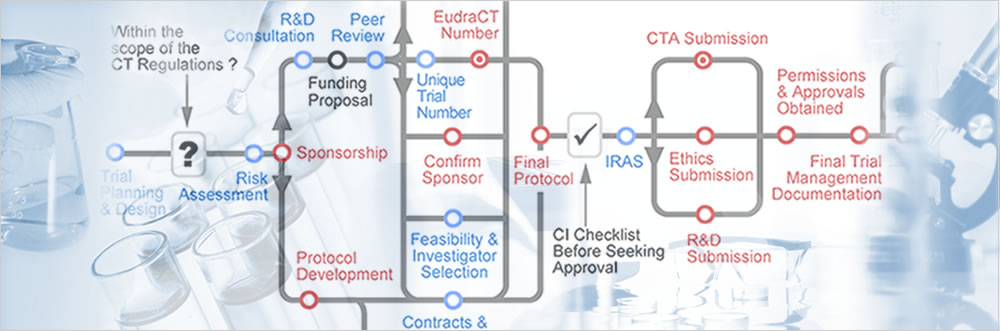
Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations | The BMJ

Ongoing Clinical Trials for the Management of the COVID-19 Pandemic: Trends in Pharmacological Sciences

Make it Public – new partnership will automatically register all UK clinical trials | NHS Research Scotland | NHS Research Scotland

PDF) Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet
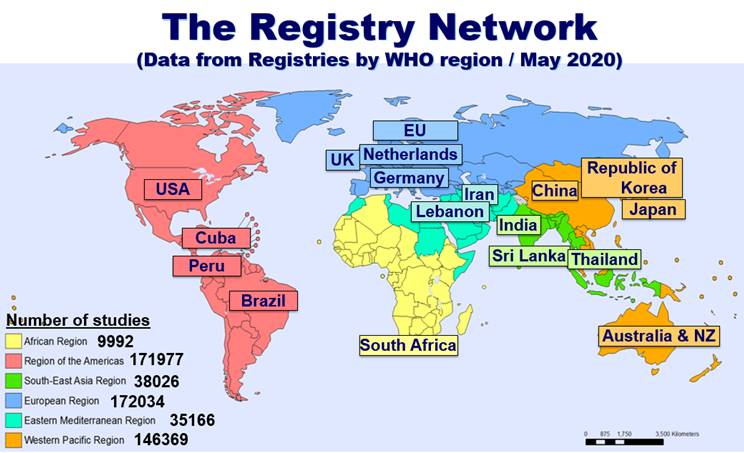
The WHO International Clinical Trials Registry Platform: Providing global clinical trial information to all - On Medicine

Patrick Wild Centre on Twitter: "Join us for our trial information evenings in May. An opportunity to find out more about the Zynerba pharmaceuticals clinical trial of cannabidiol gel for fragile X